The research paper was published (Langmuir)
Wang X, Gao Q, Li L, et al. Quantifying and Decoupling Molecular Interactions of Ionic Liquids with Gold Electrodes. Langmuir. 2024/05/28 2024. https://doi.org/10.1021/acs.langmuir.4c00688
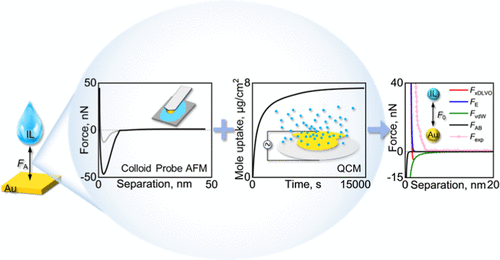
Abstract: This work combined gold colloid probe atomic force microscopy (AFM) with a quartz crystal microbalance (QCM) to accurately quantify the molecular interactions of fluorine-free phosphonium-based ionic liquids (ILs) with gold electrode surfaces. First, the interactions of ILs with the gold electrode per unit area (F′A, N/m2) were obtained via the force-distance curves measured by gold probe AFM. Second, a QCM was employed to detect the IL amount to acquire the equilibrium number of IL molecules adsorbed onto the gold electrode per unit area (NIL, Num/m2). Finally, the quantified molecular interactions of ILs with the gold electrode (F0, nN/Num) were estimated. F0 is closely related to the IL composition, in which the IL with the same anion but a longer phosphonium cation exhibits a stronger molecular interaction. The changes in the quantified interactions of gold with different ILs are consistent with the interactions predicted by the extended Derjaguin–Landau–Verwey–Overbeek theory, and the van der Waals interaction was identified as the major contribution of the overall interaction. The quantified molecular interaction is expected to enable the direct experimental-derived interaction parameters for molecular simulations and provide the virtual design of novel ILs for energy storage applications.
This study presents an experimental approach to exploring interactions between ionic liquids and gold electrode surfaces, aiding the design of novel ILs for energy storage applications.