The review article was published (Nanoscale Horiz.)
An, R.; Wu, N.; Gao, Q.; Dong, Y.; Laaksonen, A.; Shah, F. U.; Ji, X.; Fuchs, H., Integrative studies of ionic liquid interface layers: bridging experiments, theoretical models and simulations. Nanoscale Horizons 2024, 9, (4), 506-535. DOI https://doi.org/10.1039/D4NH00007B
Abstract: Ionic liquids (ILs) are a class of salts existing in the liquid state below 100 °C, possessing low volatility, high thermal stability as well as many highly attractive solvent and electrochemical capabilities, etc., making them highly tunable for a great variety of applications, such as lubricants, electrolytes, and soft functional materials. In many applications, ILs are first either physi- or chemisorbed on a solid surface to successively create more functional materials. The functions of ILs at solid surfaces can differ considerably from those of bulk ILs, mainly due to distinct interfacial layers with tunable structures resulting in new ionic liquid interface layer properties and enhanced performance. Due to an almost infinite number of possible combinations among the cations and anions to form ILs, the diversity of various solid surfaces, as well as different external conditions and stimuli, a detailed molecular-level understanding of their structure-property relationship is of utmost significance for a judicious design of IL–solid interfaces with appropriate properties for task-specific applications. Many experimental techniques, such as atomic force microscopy, surface force apparatus, and so on, have been used for studying the ion structuring of the IL interface layer. Molecular Dynamics simulations have been widely used to investigate the microscopic behavior of the IL interface layer. To interpret and clarify the IL structure and dynamics as well as to predict their properties, it is always beneficial to combine both experiments and simulations as closely as possible. In another theoretical model development to bridge the structure and properties of the IL interface layer with performance, thermodynamic prediction & property modeling have been demonstrated as an effective tool to add the properties and function of the studied nanomaterials. Herein, we present recent findings from applying the multiscale triangle “experiment–simulation–thermodynamic modeling” in the studies of ion structuring of ILs in the vicinity of solid surfaces, as well as how it qualitatively and quantitatively correlates to the overall ILs properties, performance, and function. We introduce the most common techniques behind “experiment–simulation–thermodynamic modeling” and how they are applied for studying the IL interface layer structuring, and we highlight the possibilities of the IL interface layer structuring in applications such as lubrication and energy storage.
This study offers an in-depth examination of ionic liquid interface layers, seamlessly integrating experimental data, theoretical insights, and simulation results to inform the strategic selection of electrolytes and the optimization of anode and cathode materials.
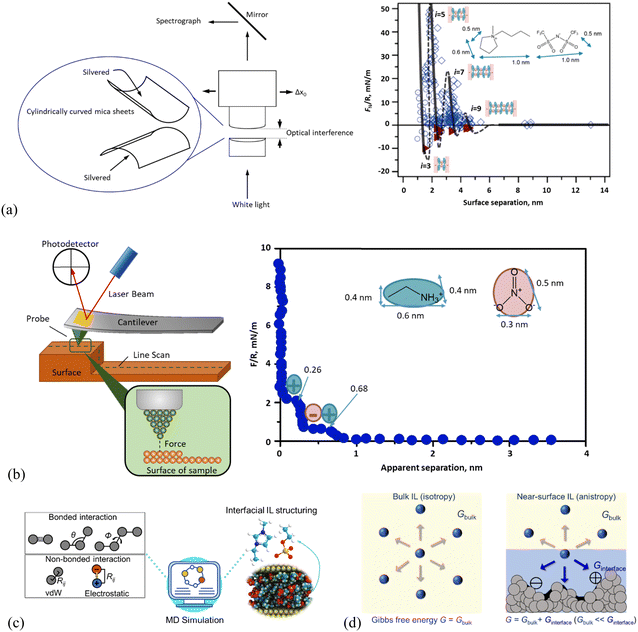
Figure. Schematic presentation of the working principle in (a) surface force apparatus (SFA) and a typical SFA-measured force curve for [C4C1Pyrr][NTf2] confined between mica sheets, (b) atomic force microscopy (AFM) and AFM-measured normal force curve for EAN on mica with a 5 μm-radius silica colloid probe. In the right panel of (a), open diamonds and circles indicate points measured on approach and retraction of the surfaces, respectively. Filled triangles in (a) indicate points measured from the jump-apart of the surfaces from an adhesive minimum. i means the number of ion layers. The lines are a guide to the eye to show the oscillatory nature of the forces, with solid lines through measured regions and dashed lines through the regions inaccessible to measurement due to the jump-in from the previous energy barrier. (c) Molecular dynamics (MD) simulation method for detailing the structure and dynamics of the ionic liquid interface layer. (d) Molecular thermodynamics modeling, here in quantitatively describing bulk and ionic liquid interface layer via Gibbs free energy (G). The solid surface in contact with the IL (right panel) shows varying nanostructured features, and/or chemical heterogeneities, and/or surface charges.
