LOHC Technology
What are LOHCs:
LOHCs are organic compounds that can absorb and release hydrogen through chemical reactions, and thus the chemically stored hydrogen in LOHCs can be transported and stored safely at room temperature.
The simplest representative LOCHs are benzene/cyclohexane, followed by toluene/methylcyclohexane, benzyltoluene/perhydro-benzyltoluene and dibenzyltoluene/perhydro-dibenzyltoluene, as well as other multi-ring systems including decalin/naphthalene, biphenyl/bicyclohexyl, etc. Among them, the aromatic toluene, benzyltoluene, and dibenzyltoluene are the most studied, and their properties are listed in the below Table.
| MCH/TOL | H12-BT/H0-BT | H18-DBT/H0-DBT | ||||
| Molecular formula | C7H14 | C7H8 | C14H26 | C14H14 | C21H38 | C21H20 |
| H2 storage density at 20 °C/kg-H2 m-3 | 47.4 | 0 | 54.5 | 0 | 57.2 | 0 |
| Molecular weight/g mol-1 | 98.189 | 92.141 | 194.357 | 182.261 | 290.54 | 272.384 |
| Isomeric peaks in GC | 1 | 1 | 6 | 3 | >20 | >12 |
| GHS labels | 2, 7, 8, 9 | 2, 7, 8 | 7, 8 | 7, 8, 9 | n.a. | 8, 9 |
| Maximum diameter of the molecules/nm | 0.60 | <0.60 | 1.15 | <1.15 | 1.5 | <1.5 |
| Phase at 290 °C and 3 barabs | Vapor | Vapor | Liquid | Liquid | Liquid | Liquid |
| Dyn. viscosity at 20 °C/mPa S | 0.5 | 0.6 | 7 | 4 | 49 | 424 |
| Melting point at atm/°C | -126 | -95 | -80-70 | -30 | <-50 | -34 |
| Boiling point at atm/°C | 101 | 111 | 264-272 | 277-290 | »371 | »392 |
| Density at 20 °C/kg m-3 | 770 | 870 | 876 | 996 | 913 | 1044 |
| DrH0 at 25 °C per mole H2/kJ mol-1 | +68.3 | -68.3 | +63.5 | -63.5 | +65.4 | -65.4 |
Why LOHCs:
LOHC is mostly fuel-like and can be integrated into the existing fuel infrastructure with slight modifications without building a completely new one. In addition, LOHC does not pose a higher risk potential than known fuels.
Compared to other storage media, such as n-ethyl-carbazole, toluene, dibenzyl toluene, ammonia, and methanol, LOHCs can be used multiple times like aromatics (closed systems), instead of only once (MeOH, NH3…).
Compared to other storage options: LOHCs possess high volumetric hydrogen storage density (>50 g-H2/l), which is much higher than compressed hydrogen gas (20 – 30 g-H2/l). LOHCs technology is recognized to be excellent for long-distance and large-scale hydrogen storage and transportation due to its high hydrogen storage capacity, environmental friendliness, safety, and efficiency.
LOHC process:
LOHCs technology is based on reversible hydrogen storage and release reactions, i.e., hydrogenation and dehydrogenation. The fundamental principle is shown in the Figure. The hydrogenation process is an exothermic reaction and carried out at elevated pressures (approx. 30-50 bar) and temperatures of approx. 150-200°C in the presence of a catalyst. During this process, the organic hydrogen storage liquid is mixed with raw hydrogen in the reactor to form the corresponding saturated compound, which can be stored or transported under ambient conditions.
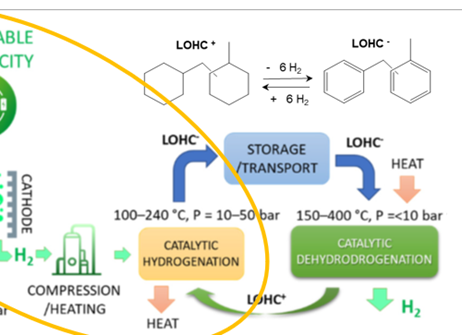
The dehydrogenation process is an endothermic reaction and takes place at elevated temperatures (250-320°C) in the presence of a catalyst. During this process, hydrogen is extracted from the formed saturated compound, and LOHC is thus regenerated that can be stored and transported back to the hydrogenation station.
State-of-the-art:
The Japanese company Chiyoda Corporation has rewarded this LOHC concept, and, in collaboration with other partners, it has demonstrated the feasibility of the first transport route via Brunei to Japan ().
Industrial development by the German company Hydrogenious LOHC Technologies pushes the commercialization of benzyl-toluene as the hydrogen carrier, and several projects have been implemented to demonstrate the feasibility of this LOHC technology.
Beyond the state-of-the-art (electrocatalytic hydrogenation):
LOHC electrochemistry presents compelling perks over its thermal chemistry. The utilization of electrode potentials overcomes the thermodynamics of endothermic reactions and promotes kinetics, allowing operations at ambient pressure and temperature. Electrification of LOHC chemistry can not only enhance energy efficiency but also enable the direct use of LOHCs in electrical energy storage devices, representing a significant improvement over conventional thermal methods. Moreover, the working principle of LOHC electrochemical cells helps minimize the need for hydrogen separation after release, enhancing the overall efficiency.
