Article 7 (Research Paper)
Zuo Z.; Lu X.; Ji X.; Modeling self-diffusion coefficients of ionic liquids using ePC-SAFT and FVT combined with Einstein relation. AIChE J. 2024;e18468. doi:10.1002/aic.18468
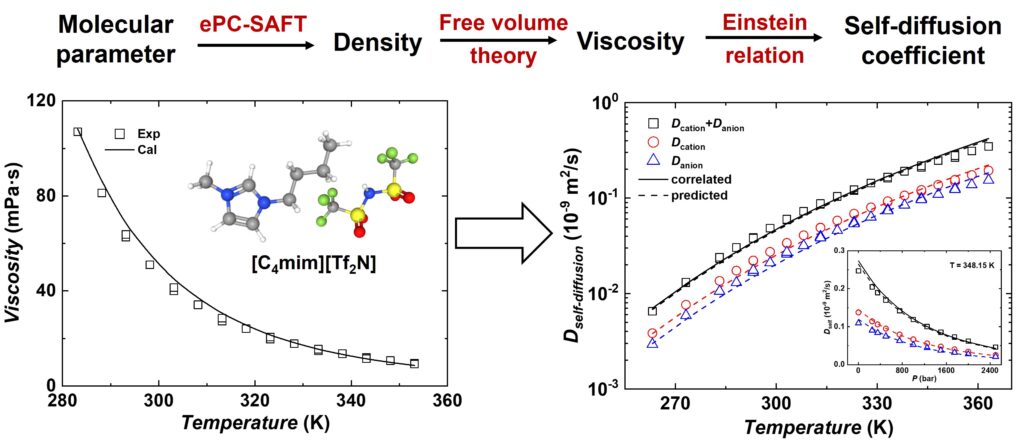
Abstract: The electrolyte perturbed-chain statistical associating fluids theory (ePC-SAFT) coupled with free volume theory (FVT) was combined with Einstein relation, that is, ePC-SAFT-FVT-E, to describe self-diffusion coefficients (SDCs) of ionic liquids (ILs). In modeling, ePC-SAFT was used to calculate density, while FVT parameters, determined from viscosity data, were utilized to calculate the summation of ionic SDCs through the Einstein relation with one parameter. Two strategies were employed to determine this parameter: fitting experimental data for each IL or estimating a universal parameter from van der Waals volume. Comparative analysis reveals good agreement with experimental data, with average absolute relative deviations (ARDs) of 8.14% (strategy 1) and 10.29% (strategy 2). Subsequently, cationic and anionic SDCs were reliably determined from the summation of ionic SDCs, with average ARDs of 10.80% and 10.21%, respectively. This study indicates the ePC-SAFT-FVT-E model, employing viscosity-derived parameters and three universal parameters, reliably predicts SDCs of ILs in wide temperature and pressure ranges.