The research paper was published (JCED)
Zhida Zuo, Bei Cao, Linghong Lu, Xiaohua Lu, Xiaoyan Ji. Thermodynamic Study of Ionic Liquids 1-Hexyl-3-methylimidazolium Halide with Methanol Mixtures. J. Chem. Eng. Data 2024. https://doi.org/10.1021/acs.jced.3c00707
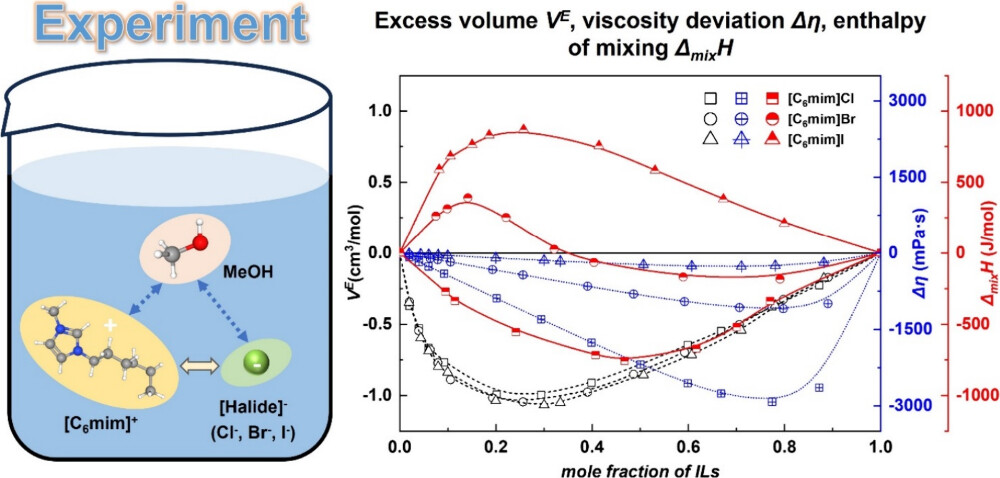
Abstract: To investigate the effects of anion size, solvent content, and temperature on their physical properties, in this work, densities and viscosities of three binary mixtures, including ionic liquids (ILs) composed of the cation 1-hexyl-3-methylimidazolium (i.e., [C6mim]+) and the halide anions (i.e., Cl–, Br–, and I–), along with methanol (MeOH), were measured across the entire concentration range at temperatures from 288.15 to 323.15 K, and enthalpies of mixing were determined at temperatures of 298.15 and 308.15 K. Excess volumes and viscosity deviations were calculated to study the nonideal behavior of the mixtures. The results of excess volumes suggest that a combination of intermolecular interactions and packing effects contributes to their nonideal behaviors, with packing effects likely dominating the negative excess volume. Viscosity deviations and enthalpies of mixing indicate that MeOH molecules intend to coordinate with IL ions (IL cations and IL anions) in the IL-rich region, with stronger interactions observed between [C6mim]Cl and MeOH compared to ([C6mim]Br + MeOH) and ([C6mim]I + MeOH) mixtures. Additionally, the nonrandom two-liquid model and the Gibbs–Helmholtz equation were combined to describe the enthalpies of mixing for the studied mixtures reliably.
This study provides experimental measurements to explore the physicochemical properties of ionic liquids and co-solvents, investigating their mechanisms within the system. These findings contribute to the development of innovative electrolytes for the EPOCH project.